The modern air conditioning system cools an indoor space by using the refrigeration cycle. This refrigeration cycle works by controlling the level of energy in the system’s refrigerant: Some parts of the system have energy-packed refrigerant that is ready to release heat, whereas other parts have energy-depleted refrigerant that is ready to absorb heat.
The Four Core Components
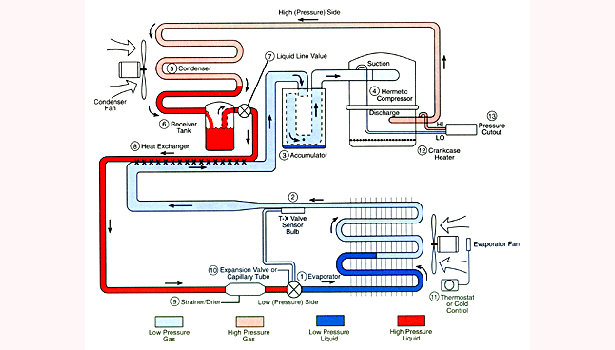
Four core components (the compressor, the condenser, expansion valve, and the evaporator) work together to control when/where refrigerant is absorbing heat, and when/where it is releasing heat.
The Compressor
Imagine a massive pipe filled with rushing steam. The steam enters into a machine, which then turns on and rumbles. When the steam leaves the machine (in a different pipe than it came in through), the steam is extremely high-pressure, moving extremely fast. Low-pressure water vapor enters the machine, and high-pressure water vapor leaves.
An air conditioning compressor works the same. It takes low-pressure gas refrigerant coming into it, and turns it into high-pressure gas by the time the refrigerant leaves, increasing the chemical’s temperature as well.
A refrigeration system needs to be pressurized. Take away the compressor, and the refrigerant just sits in the air conditioner without moving.
The Condenser
Upon leaving the compressor, the high-pressure gas refrigerant enters the condenser, where the gas becomes a liquid.
Picture a twisting coil of pipes that is pumped full of hot, pressurized water vapor. And imagine that these pipes are so long, that by the time the water vapor exits the pipe, the water has cooled down and become a liquid. It’s still pressurized — the liquid water is shooting through the pipes — but it has lost a lot of its heat to the outside air. The air around it is hot from stealing the energy from the water.
This is how a condenser works. The condenser is a network of pipes that a hot, gas refrigerant passes through. By the time it leaves the condenser, the refrigerant has lost a lot of its heat and is now a liquid.
This component works due to the interaction between pressure and temperature. For example, in a chef’s pressure cooker, the pressure increases as the temperature of the food inside does. However, this is also true in reverse. If you increase the pressure, the temperature will increase in response. If you decrease the pressure, temperature decreases.
So, a condenser decreases the pressure of the refrigerant, which then causes the temperature to decrease. And a refrigerant getting colder means that the air around will be getting warmer. In an air conditioner, the condenser expels hot air — air that has stolen its energy from the refrigerant.
The Expansion Valve/Capillary Tube
The refrigerant has left the condenser as a high-pressure liquid. A high-pressure refrigerant, just like high-pressure water, wants to shoot through the pipes really fast. However, for the evaporator to do its job, the refrigerant has to move slower, and be lower pressure. This is something like a dam in a fast-moving river — the water wants to move faster, but the dam slows it down and regulates the amount of water that passes through.
This is the same with the expansion valve/capillary tube. Both perform the same function of slowing the refrigerant down. A capillary tube does this by “damming” up the refrigerant — the pipe leaving the tube is smaller than the pipe entering it. A thermostatic expansion valve performs the same job in a slightly different way (a spring, affected by the temperature of the refrigerant, regulates how much of the refrigerant gets through).
The Evaporator
The evaporator does the exact opposite of the condenser. Instead of turning the refrigerant from gas to liquid, it turns the refrigerant from liquid to gas (thus the name “evaporator”). Instead of expelling heat, it absorbs heat and turns the air cold around it. This is where the “conditioning” of the air occurs.
Continuing the pipe example, imagine a long series of pipes in an extremely hot room. When the water first enters the pipes, it is moving as a slow liquid. But by the end, after many twists and turns, the pipe and water have absorbed so much heat that the water has boiled and is a low-pressure gas.
Think of the pressure cooker, remembering that pressure increases as temperature increases. Again, the reverse is true: increasing (or decreasing) the pressure will cause the temperature to change. So the evaporator and condenser work not by using a machine to heat/cool the refrigerant, but by changing the pressure of the refrigerant, which thus changes its temperature. In the evaporator, the refrigerant is evaporated into a gas, which forces it to absorb heat from the air around it, cooling the house where the system is placed.
Back to the Compressor
After this, the refrigerant returns to the compressor as a gas. The compressor takes the gas and pressurizes it, and the cycle starts over. In our pipe/water example, the steam returns to the machine, where it is converted again into high-pressure, fast-moving water vapor.
The Magic of Refrigerants
The use of refrigerants are critical to the success of an air conditioning system. Refrigerants work because of how cold they are. The analogy of hot water running through pipes might give readers the impression that the refrigerants are white-hot when rushing through an air conditioning system. But this is not case. Rather, they are extremely cold.
For example, one refrigerant, R-410, has a boiling point of about minus 55° F (or minus 48.5°C), though this changes as the pressure of the refrigerant changes. This is why an entire network of pipes isn’t needed to convert refrigerant between liquid and gas — room temperature itself is significantly above the refrigerant’s boiling point. The colder a refrigerant, the more heat it can absorb, and the greater its ability to cool in an air conditioning system.
So what is superheat/subcooling?
Subcooling
An expansion device/capillary tube is meant to control the amount of liquid refrigerant passing through onto the evaporator. If gas refrigerant reaches the device/tube, the system will not work as it should. To ensure only liquid refrigerant reaches the component, the refrigerant is subcooled. Simply put, the refrigerant is made significantly cooler than the boiling point, to make sure all of the refrigerant is in liquid state.
For example, if refrigerant needs to be under 100°F to be a liquid, the refrigerant may be cooled to 85°F. This ensure that there are no pockets of refrigerant left that are accidentally over 100°F, which could prevent the correct operation of the system.
Superheat
Superheat is similar. A compressor is made to compress gas refrigerant. It can be damaged if liquid refrigerant flows into it. So the gas is heated significantly above boiling point, to ensure there are no pockets of liquid refrigerant left. So if the refrigerant needs to be heated over 100°F to be a gas, the system will heat it to 115°F, just to be sure.


Report Abusive Comment