The first point to understand about refrigeration theory is that heat is energy, and it can be made to move. If enough heat is removed from a glass of water, the water will freeze to ice. When that heat is allowed to move back into the ice, the ice will melt.
Heat has its own laws, called the laws of thermodynamics.
One of those laws is that heat will move from a place that has a lot of heat to a place that has less heat. Another way to put it is that heat will move from a place of higher intensity to a place of lower intensity. It happens naturally, and automatically, similar to the way the north pole of a magnet is attracted to the south pole of another magnet.
So, to take advantage of this point of the theory, air conditioning and refrigeration equipment is designed to create a cold area that acts as a "heat sponge" to soak up heat from the surrounding air or food. The heat is then moved to a place where it can be released safely and efficiently.
The second main point to understand about refrigeration theory has to do with why we use evaporators and condensers.
When a liquid like water or refrigerant absorbs enough heat to start boiling, what's happening is that the added heat energy causes the vibration of the liquid's molecules to speed up to the point where they move farther apart from each other. When the molecules of liquid reach a certain distance from each other, the liquid changes into a vapor. This is called boiling, evaporating, or vaporizing.
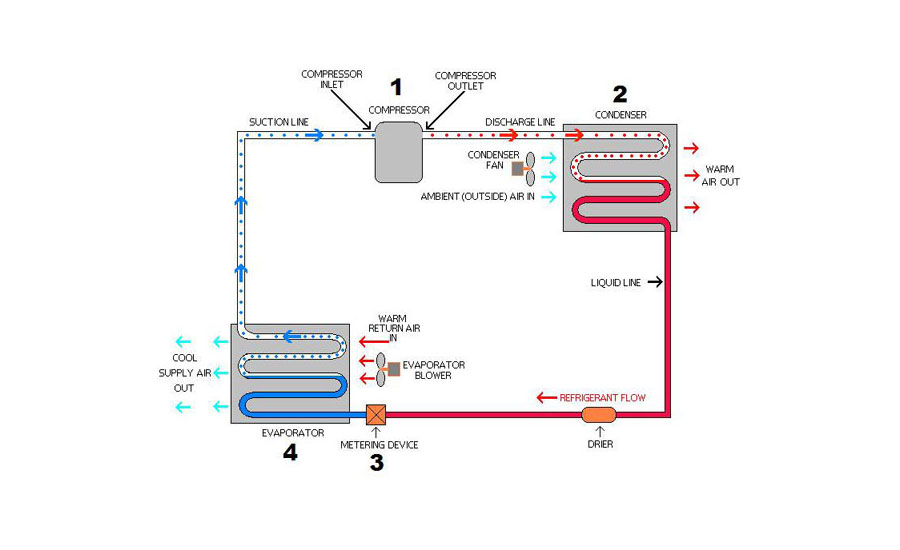
A liquid absorbs intense levels of heat as it changes state to a vapor. Air conditioning and refrigeration equipment is designed to use this point of refrigeration theory by keeping a constant flow of refrigerant vaporizing and absorbing heat in the evaporator.
So the evaporator is the "heat sponge" area, and the refrigerant vaporizing inside of it is absorbing the heat.
When vapor cools and releases enough heat energy, its molecules will slow down and move closer together to the point where the vapor changes into a liquid.
This is called condensation, and it's also a change of state (from vapor to liquid). To condense, a vapor must release the same intense level of heat that it absorbed when it vaporized.
Air conditioning and refrigeration uses this point of refrigeration theory by causing refrigerant to cool and condense in the condensing unit. One way to think of it is that the heat that the refrigerant absorbed in the evaporator or "heat sponge" area is squeezed out of the refrigerant in the condenser.
The refrigerant repeats this cycle continuously, soaking up (absorbing) heat in the evaporator, and squeezing it out (releasing it) in the condenser.
For more information, visit www.air-conditioning-and-refrigeration-guide.com.
Publication date: 2/6/2017

Report Abusive Comment