What Is Steam Quality?
Steam quality is the proportion of saturated steam (vapor) in a saturated condensate (liquid)/steam (vapor) mixture. A steam quality of 0 indicates 100 percent liquid (condensate), while a steam quality of 100 indicates 100 percent steam. One pound of steam with 95 percent steam and 5 percent of liquid entrainment has a steam quality of 0.95.
The measurements needed to obtain a steam quality measurement are temperature, pressure, and entrained liquid content. A high percentage (88 percent or more) of industrial steam systems use saturated steam for process applications. Saturated steam (meaning steam that is saturated with energy) is completely gaseous and contains no liquid.
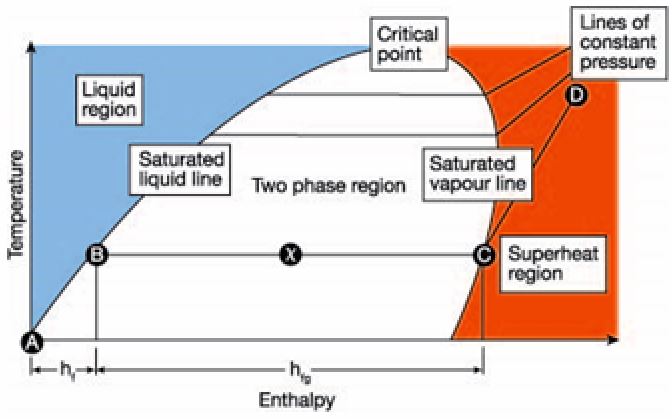
The boiler operation uses chemical energy from a fuel source to deliver energy to the boiler water. Inside the boiler, liquid gains energy from the combustion process and changes state into saturated steam. Water enters the boiler at point A, and the water gains sensible energy (hf) to point B. The change of state is referenced as point B in Figure One. As the saturated steam acquires more energy from the boiler combustion process, the steam achieves a high quality (moving left to right), as represented by points B to C. The increase in energy gained by the steam from points C to D goes toward the superheat of the vapor.
A directly proportional relationship exists between temperature and pressure in saturated steam. This means that as the temperature increases, so does the pressure. Illustrated by the lines of constant pressure in One, more sensible energy (hf) is needed to for water to transition from point A to point B and become a vapor.
When steam enters the process, the energy level goes from right to left as the process absorbs the energy from the steam.
Why Steam Quality Is Important
Today’s manufacturing techniques of heat transfer, control, and standards are all dedicated to improving and providing the highest-quality product to the marketplace. To attain the highest quality, each manufactured component of the final product is inspected repeatedly and measured for its quality. This ensures that it meets the manufacturer’s and consumer’s expectations.
Steam is a vital and critical part in producing the final product; therefore, steam quality should be one of the main measurable points in creating a product in today’s manufacturing facilities. All heat transfer components (shell/tube, plate/frame, plate/coil, tracing, etc.) base performance calculations on 100 percent steam quality, unless the manufacturer is informed by the end user that the steam quality is lower than 100 percent.
Unfortunately, steam quality is typically not monitored closely and is often assumed to be 100 percent. Therefore, issues that arise from poor steam quality are blamed on some other item in the system. Based on field documentation by SEA, a high percentage of steam systems are operating below acceptable steam quality levels.
What Are the Effects of Steam Quality?
Low steam quality affects steam system operations in many ways. Here are four:
- Reduced heat transfer efficiency: The major problem with low steam quality is the effect on the heat transfer equipment and process. In some cases, low steam quality can reduce heat transfer efficiency by more than 65 percent. The liquid entrained in the steam has sensible energy (16 percent estimated: varies with pressure), which has a significantly lower amount of energy than the steam vapor’s latent energy (94 percent). Therefore, less usable energy is being delivered to the steam process equipment. Also, the additional liquid (low steam quality) collects on the wet surface of the heat exchanger, causing an additional buildup of a liquid, which reduces the ability of the steam’s latent energy to be transferred to the product.
- Premature valve failure: Liquid passing through steam control valves will erode the internals of the valves, causing premature failure.
- Internal turbine component failures: Liquid introduced with the steam in a saturated turbine operation will reduce the life expectancy of the internal components.
- Water hammer: Steam systems are usually not designed to accommodate the additional liquid in steam. Additional liquid creates the chance for water hammer to occur. Water hammer poses a safety risk and may cause premature failure in the steam system.
How To Measure Steam Quality
A true measurement of steam quality can be obtained from the use of a throttling calorimeter and Ganapathy’s steam plant calculations. Unfortunately, most industrial plants do not have the luxury or capability of doing the testing.
Another way to measure steam quality is by relying on the basics of steam. Saturated steam is a dry invisible gas and only becomes visible with entrained air or liquid. Therefore, opening a steam valve and allowing steam to be released into the atmosphere provides an estimate of the steam quality in the system.
Examples
Figure Two indicates an acceptable steam quality. The discharge from the valve through the tube is almost invisible.
Figure Three shows the discharge from the valve off the steam line to be very visible, with liquid being discharged with the steam vapor.
The steam quality is not acceptable for the process.
Figure Four shows the discharge from the valve off the steam line to be very visible, with liquid being discharged with the steam vapor.
The steam quality is not acceptable for the process.
Calorimeter
The apparatus used to determine the moisture content of steam is called a calorimeter. However, since it may not measure the heat in the steam, the name does not describe the function of the apparatus. The first name used was the “barrel calorimeter,” but the liability of error was so great that its use was abandoned.
Modern calorimeters are generally of either the throttling or separator type.
Throttling Calorimeter
Figure Five shows a typical form of throttling calorimeter. Steam is drawn from a vertical main through the sampling nipple. Then it passes around the first thermometer cup, through a ⅛-in. orifice in a disk between two flanges, around the second thermometer cup, and to the atmosphere.
The instrument and all pipes and fittings leading to it should be thoroughly insulated to diminish radiation losses. Care must be taken to prevent the orifice from becoming blocked with corrosion materials. The discharge piping should be short to prevent backpressure below the disk.
When steam passes through an orifice from a higher to a lower pressure, as is the case with the throttling calorimeter, no external work has to be done in overcoming a resistance. Hence, if there is no loss from radiation, the quantity of heat in the steam will be exactly the same after passing the orifice as before passing.
If the higher steam pressure is 160 psig and the lower pressure that of the atmosphere, the total heat in a pound of dry steam at the former pressure is 1,195.9 Btu/lb. and is 1,150.4 Btu/lb. at the discharge pressure, for a difference of 45.4 Btu/lb. As this heat/energy will still exist in the steam at the lower pressure, since there is no external work done, its effect must be to superheat the steam.
Assuming the specific heat of superheated steam to be 0.47, each pound passing through will be superheated 45.4⁄0.47 = 96.6°.
If, however, the steam had contained 1 percent of moisture, it would have contained less heat units per pound than if it were dry. Since the latent heat of steam at 160 psig is 852.8 Btu/lb., it follows that the one percent of moisture would have required 8.5 Btu/lb. to evaporate it, leaving only 45.4 – 8.5 = 36.9 Btu/lb. available for superheating; hence, the superheat would be 36.9⁄0.47 = 78.5°, as against 96.6° for dry steam. In a similar manner, the degree of superheat for other percentages of moisture may be determined. The action of the throttling calorimeter is based upon the foregoing facts, as shown below.
| H | = | total heat of one pound of steam at inlet steam pressure |
| L | = | latent heat of steam at inlet steam pressure |
| h | = | total heat of steam at reduced pressure after passing orifice |
| t1 | = | temperature of saturated steam at the reduced pressure |
| t2 | = | temperature of steam after expanding through the orifice in the disc |
| 0.47 | = | the specific heat of saturated steam at atmospheric pressure |
| x | = | proportion by weight of moisture in steam |
The difference in Btu/lb. of steam at the boiler pressure and after passing the orifice is the heat available for evaporating the moisture content and superheating the steam. Therefore,
H – h = xL + 0.47 (t2 –t1)
or
x = H – h – 0.47 (t2 –t1)
L
Almost invariably, the lower pressure is taken as that of the atmosphere. Under such conditions, h = 1,150.4 and t1 = 212°F.
A slight error may arise from the value used as the specific heat of superheated steam at atmospheric pressure: 0.47. However, this value is very nearly correct, and any error resulting from its use will be negligible.
Compact Throttling Calorimeter
There are many forms of throttling calorimeter, all of which work upon the same principle. This calorimeter consists of two concentric metal cylinders screwed to a cap containing a thermometer well. The steam pressure is measured by a gauge placed in the supply pipe or other convenient location. Steam passes through the orifice A and expands to atmospheric pressure, its temperature at this pressure being measured by a thermometer placed in the cup C. To prevent radiation losses as far as possible, the annular space between the two cylinders is used as a jacket, steam being supplied to this space through the hole B.
The limits of moisture within which the throttling calorimeter will work are, at sea level, from 2.88 percent moisture at 50 pounds gauge pressure and 7.17 percent moisture at 250 pounds pressure.
Separating Calorimeter
The separating calorimeter mechanically separates the entrained water from the steam and collects it in a reservoir, where its amount is either indicated by a gauge glass or where it is drained off and weighed. Figure 6 shows a calorimeter of this type.
The steam passes out of the calorimeter through an orifice of known size so that its total amount can be calculated or it can be weighed. A gauge is ordinarily provided with this type of calorimeter, which shows the pressure in its inner chamber and the flow of steam for a given period, this latter scale being graduated by trial.
The instrument, like a throttling calorimeter, should be well insulated to prevent losses from radiation.
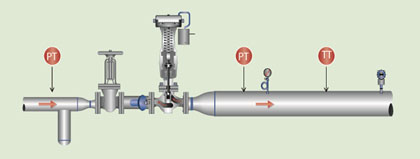
Steam Pressure Reducing: Testing Steam Quality
A steam pressure-reducing valve will work the same as a throttling calorimeter. A typical installation of a steam pressure-reducing station, by adding pressure measurement upstream and downstream with the addition of temperature measurement downstream, provides the continuous online “calorimeter.”
Road Map To Ensure a High Steam Quality
Taking the following six steps will ensure a high steam quality:
- Insulate steam lines and components.
- Establish proper steam line drip leg steam trap stations.
- Develop proper start-up procedures.
- Implement a proactive boiler chemical program.
- Implement a proactive steam system management program.
- Install steam separators (mechanical coalescing design), if needed.
This content was provided by Inveno Engineering LLC.
Submit your own guest content here!





Report Abusive Comment