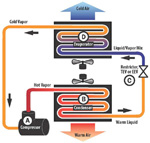
Figure 1. Basic system. (Click on the image for an enlarged view.)
The refrigerant vapor is cooled to the point of condensation to a liquid because its pressure remains high (C) but its temperature is reduced. The liquid refrigerant is expanded into the evaporator (D) at a low pressure which allows it to boil at a low temperature. The temperature of the evaporator is designed to be lower than the air it is cooling, so heat from the air will flow into the cold refrigerant. The cold vapor returns to the compressor to start the cycle over. Note that in all cases, the heat flow is from the hotter to the cooler, just as the Second Law requires.
SENSIBLE vs. LATENT HEAT
It is obvious that adding heat to a substance will raise its temperature and subtracting heat will lower its temperature. Less obvious is that the amount of heat needed to do so is relatively small. For water, it is one Btu per pound for each degree F change in the water temperature. Copper, a good conductor, only requires one-twelfth of the heat to change one degree; in other words, the same amount of heat will change the temperature of a pound* of copper by 12°F. Since the copper or water, in these examples, did not change state, the heat required to change the temperature is called sensible heat.[*For the purposes of this discussion, and to simplify the text, temperature will be expressed in degrees Fahrenheit (°F), and weight as pounds (lbs). For a true measure of heat quantities, mass is used for calculation rather than weight.]
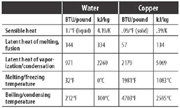
Table 1. Latent heat comparison - water vs. copper. (Click on the table for an enlarged view.)
Table 1 shows the comparison between water and copper.
Note that the amount of heat to melt copper is less than that needed to melt ice, but the temperature is very different.
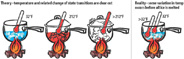
Figure 2. Change of state - theory vs. reality. (Click on the image for an enlarged view.)
In the real world, the transitions are never so clear cut (Figure 2).
The water in the glass can begin to warm even when ice is still present, but the amount of heat needed to melt the ice is still 144 Btu/lb.
Questions
1) What is the First Law of Thermodynamics?2) Define Btu.
3) What are the three common states of matter?
4) What is the difference between sensible and latent heat?
5) What does SI refer to, and what temperature scales are used in it?
6) If a cold object and a hot object are in contact with each other, can the cold object transfer even more of its heat to the hot object making it hotter?
Going Further
This presentation is meant as an easy to understand overview. It is accurate for basic understanding but leaves out the math and equations.The Part 1 figure concerning the stack of blocks is accurate in common experience but not at the sub-atomic or quantum level. In the realm of the very small, entropy and energy can flow the “wrong” way.
Sources
Modern Refrigeration and Air ConditioningAlthouse, Turnquist and Bracciano - Goodheart Wilcox Publishing, Tinley Park, IL
Provides easy-to-use formulas and illustrations of thermodynamics and HVACR systems. Available from Amazon.com and other technical booksellers.
ASHRAE Fundamentals Handbook
American Society of Heating, Refrigerating and Air-Conditioning Engineers, 1791 Tullie Circle, N.E, Atlanta, GA 30329; www.ashrae.org
Provides in-depth engineering data, with complete formulas, for HVACR applications.
Web searches for “thermodynamics” and “entropy” will yield thousands of sources for more information, although few are focused on HVAC applications.
Answer to the puzzle in Part 1- “What is the temperature of outer space?” A trick question because answers can vary with frames of reference. Outer space is a near vacuum and therefore has no temperature at all; however, there are energetic sub-atomic particles that have “temperatures” in the millions of degrees. The temperature of the background radiation is about 3 degrees above absolute zero. Remember that heat always moves from a higher temperature to a colder temperature and can move by radiation. Surfaces facing the sun on Mercury can reach 800°F while the surface away from the sun radiates almost all its energy away and can reach temperatures of -334°F.
This article is reprinted with permission from the KE2 Therm Solutions Inc. white paper “Basic Thermodynamics for Refrigeration and Air Conditioning - Part 2.”
Publication date:12/06/2010

Report Abusive Comment