By definition, zeotropic refrigerants are blends of two or more components whose equilibrium vapor-phase and liquid-phase compositions are different at given pressures. A couple of issues that arise with these refrigerants deal with fractionation and glide.
Fractionation is “the separation of a liquid mixture into parts by the preferential evaporation of the more volatile component,” according to ASHRAE. Glide is “the temperature range that the most volatile component to the least volatile component changes state from liquid to vapor.”
Fractionation
Blended refrigerants must be charged in a liquid state. If charged in vapor state, the resulting composition in the system and the cylinder has been compromised.With the components changing state over a range of temperatures (glide), the effectiveness of the coil surface is increased. In the context of a leak, fractionation only occurs when liquid and vapor are present together and vapor leaks.
The 400 Series
ASHRAE-designated 400 Series refrigerants, such as 404A and 410A, are zeotropes. When a zeotropic refrigerant is moving through the evaporator and condenser, it has an unequal phase change. One of the original concerns with zeotropic blends was that if a system leaked, the result could be a change in the component percentage of the blend.After several years of testing and field experience, it has been demonstrated that blends will not separate easily through system leaks, and — for the most part — if it’s going to occur, it will take place with a system that has laid dormant and leaked over a very long period of time. Therefore, topping off a leaking system with zeotropes has become a common service practice.
Glide
The temperature glide of a refrigerant is measured from the point in the evaporator or condenser at which the component of the blend with the highest vapor pressure and lowest boiling point changes state, to the component with the lowest vapor pressure and highest boiling point changes state.The operational glide of a blend has no adverse effects on system performance (with the exception of flooded evaporator or centrifugal systems). It does, however, directly relate to the increase in system efficiency.
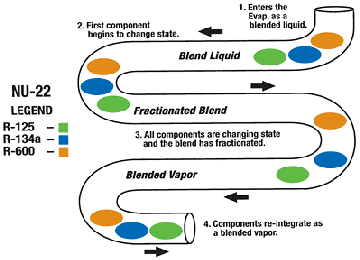
System Charging
The process of system charging zeotropes starts with evacuation. Pull a vacuum with a two-stage vacuum pump. A deep vacuum is necessary to remove moisture and noncondensables that can damage a system. Unless moisture is eliminated, hydrolysis may create acids and other harmful byproducts.Industry standards specify a 500-micron vacuum. An accurate determination of vacuum cannot be determined with a gauge set alone; it requires the use of an electronic vacuum gauge (micron).
All 400 Series ASHRAE-designated blends must be charged (leave the cylinder) in a liquid state. Charging by vapor will cause the blend to fractionate in the cylinder, creating an improper composition in the system and cylinder. This may result in decreased system performance and oil return problems.
In general, current alternatives to R-22 charge relative to the refrigerant’s volume and density. However, consult the manufacturer for charging specifics.
Once the system has stabilized, check the operational pressures, temperatures, and amp draws. Verify the superheat and subcooling readings. Adjust the charge if necessary, then record the final reading and document the amount of charge.
Two Terms
A couple of terms to remember in the context of zeotropes are “bubble point” and “dewpoint.”As heat is absorbed by the saturated liquid, the temperature increases until the vapor of the most-volatile component begins to form. This establishes the bubble point.
As heat is removed from the saturated vapor to the point that the least volatile component forms liquid droplets, that establishes the dewpoint.
The Noncondensable Test
If you are concerned that a blended refrigerant you have recovered from a leaking system has lost its integrity, you can perform a simple noncondensable test to help determine if this has occurred.Note: Before testing, make sure the recovered blend is at ambient temperature. If necessary, direct a fan across the cylinder to expedite the process.
1. Connect the high-side gauge on your manifold to the liquid port of the recovery cylinder.
2. Measure the liquid temperature of the recovered refrigerant.
3. Compare your gauge reading and liquid temperature to the corresponding bubble point reading on your pressure and temperature (P/T) chart.
4.a. If the pressure is within 5% of the P/T conversion (i.e., at 80 degrees F the pressure is 149.16 psig), the blend is acceptable, and you can recharge the system with the gas and top it off.
4.b. If the pressure is outside the 5% allowable range, return the refrigerant to the distributor for proper disposal.
A higher-than-normal reading can also reflect the presence of air in the cylinder. You can test this by slightly opening the vapor side of the cylinder to let the air escape. Then close the vapor valve and repeat the test.
Hale is tech support supervisor and McKinney is national sales director for ICOR International, based in Indianapolis. More information may be obtained at www.icorinternational.com.
Publication date: 02/03/2003

Report Abusive Comment